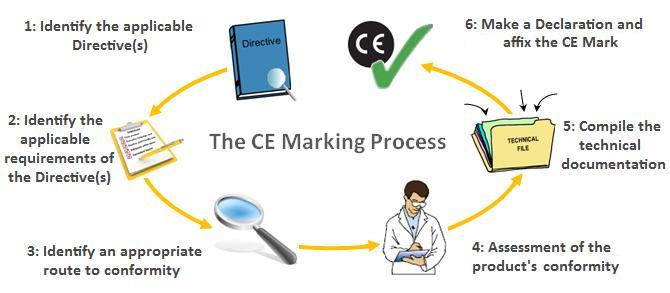
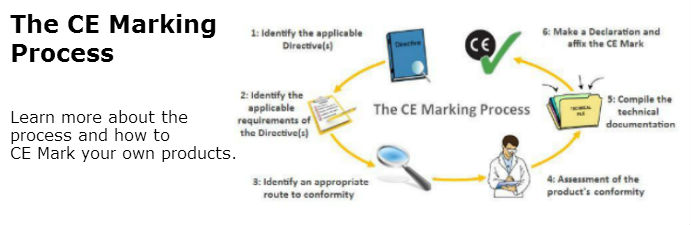
It is not a quality indicator or a certification mark the ce marking is also found on products sold outside the eea that have been manufactured to eea standards.
Ce mark medical device labeling requirements.
It shows that the device is fit for its intended purpose stated and meets.
Ce mark locations include ce mark.
Ce marking applies to products ranging from electrical equipment to toys and from civil explosives to medical devices.
The directives outline the safety and performance requirements for medical devices in the european union eu.
Ce marking is the medical device manufacturer s claim that a product meets the essential requirements of all relevant european medical device directives.
Device advice introduction to labeling requirements for medical devices including advertising over the counter exemptions in vitro diagnostics investigational devices quality system.
Ce marking is an administrative marking that indicates conformity with health safety and environmental protection standards for products sold within the european economic area eea.
Class iia medical devices.
Ce mark medical devices labeling requirements.
There are 4 classes class i class iia class iib and class iii.
The full list of these product categories is below.
Medical devices of class iia could be such as surgical gloves hearing aids diagnostic ultrasound machines etc.
Each medical device is classified into the risks involved.
The retail sales packaging.
The full list of these.
Ce marking routes of class i medical devices.
Patients should use them for a short term period any less than 30 days.
This makes the ce marking recognizable.
A ce mark is a logo that is placed on medical devices to show they conform to the requirements in the directives.
There are two major areas of confusion about the translation requirements when ce marking a product for export to the eu states.
European union ce marking the mark must be accompanied by the id of the notified body when conformity assessment procedures are required.
The medical device ce marking process will change when europe s new medical device regulation mdr 2017 745 comes into force in may 2021.
The ce mark is a legal requirement to place a device on the market in the eu.
The obl also should review the oem s essential requirements checklist declaration of conformity and ce marking certificates if notified body involvement is necessary for ce marking.
The device or its sterile package.
Prepare a declaration of conformity doc which states that your device complies with the appropriate directive.
Eu foreign language labeling requirements.